Evaluating novel NOD2 agonists in patients with Crohn’s disease
Overview:
The objective of this study was to evaluate new candidate medicines for Crohn’s disease which target the NOD2 protein using monocytes isolated from volunteers.
Imhotex engaged RxCelerate to run the study, providing the complete package, from obtaining ethical approval, through patient recruitment, managing patient visits, conducting cell-based and immunoassays, data processing and study reporting. The study was completed on-time and for a fixed price (except for pass-through costs). This case study describes the process around setting up the study and some of the key results obtained.
Background
Crohn’s Disease is a chronic inflammatory condition that can affect any part of the digestive tract, most commonly the small intestine and colon, with symptoms including pain, diarrhoea, fatigue and weight loss. It’s often diagnosed in young adults, and consequently the impact on quality of life for patients can be long-lasting. Patients are typically treated with anti-inflammatory agents, but despite this a significant proportion of patients require surgery (typically resection of affected bowel segments).
Our client, Imhotex, is a virtual drug development company founded in 2020 to develop a treatment for Crohn’s disease. They are funded by Medicxi, a European investment firm focused on the life sciences sector and managed by a small experienced team of drug development professionals. Imhotex are developing a treatment for Crohn’s disease that is based on the activation of NOD2.
NOD2 and Crohn’s disease
NOD2 (or nucleotide-binding oligomerisation domain-containing protein 2) is a protein that is part of the innate immune system and helps recognise bacteria in the gut. The gene encoding NOD2 is one of the first genes linked to Crohn’s disease through genome-wide association studies.
NOD2 encodes an intracellular “pattern recognition” receptor that recognises MDP or muramyl dipeptide, which is a small peptidoglycan and a component of bacterial cell walls. This helps the body mount a rapid, non-specific response to bacteria, especially in the gut where the microbial load is high.
Multiple mutations of NOD2 have been found to be associated with Crohn’s disease, with individuals having one of the three most common NOD2 variants having 2-4-fold increased risk of Crohn’s disease. While a highly significant association, only 35-40% of patients with Crohn’s disease have a NOD2 variant, and although around 10-15% of the European population have mutations in NOD2, most do not develop Crohn’s disease. This highlights the multifactorial nature of the disease, with other genes, the environment (such as smoking and diet) and the microbiome all interacting.
Many NOD2 variants have impaired function, perhaps in their recognition of MDP or in dimerization of NOD2, which may lead to changes in the downstream signalling cascade and alteration of other aspects of the immune system in the gut. Imhotex aim to treat patients with Crohn’s disease with activators of NOD2, to restore a more normal early immune response, helping to correct the root cause of dysregulated inflammation in patients with Crohn’s disease.
Study Objective
To evaluate new candidate medicines which target the NOD2 protein using monocytes isolated from volunteers with Crohn’s disease.
Imhotex asked us to design and run a bespoke experimental research clinical study to evaluate their two families of novel compounds which target the NOD2 protein using blood-derived monocytes from volunteers with Crohn’s disease. Monocytes are known to express NOD2 and respond to MDP by producing the pro-inflammatory cytokine IL-6.
In brief the study plan developed during discussions with Imhotex can be summarised as:
- Identify and recruit volunteers into five different subject groups:
- control patients with no NOD2 mutations
- patients with Crohn’s disease with no NOD2 mutations and
- three additional groups each comprised of patients with Crohn’s disease with one of the three main NOD2 mutations associated with Crohn’s disease
- Call volunteers into the RxCelerate site where they provide a blood sample, for monocyte isolation.
- Treat monocytes with natural or Imhotex compounds designed to activate NOD2, and measure the IL-6 response.
Study set-up and management
RxCelerate identified the NIHR Bioresource as the participant identification site and applied to them to access their database. We also prepared and submitted the appropriate documentation to the HRA NHS Research Ethics Committee (REC), obtaining ethical approval for study to commence.
The NIHR Bioresource searched their database for subjects with Crohn’s disease with various NOD2 genotypes, and matched controls. They contacted individuals from their database and shared details of those interested in participating in the study with RxCelerate. NIHR Bioresource kept the subject group identities blinded and retained the blinding list until the study database was locked at RxCelerate.
Study visits
RxCelerate study managers arranged study visits with volunteers, balancing for age and sex where possible, and ensuring that recruitment from each group was spread evenly across the study period.
Volunteers attended the RxCelerate site on the Babraham Research Campus in Cambridge, where our study team took informed consent and collected background information. Blood samples were drawn from study subjects by our trained phlebotomists, and passed, fully anonymised, to our in-house scientists for testing.
All blood sample processing was carried out at RxCelerate labs:
- Monocytes were isolated from volunteer blood samples
- Cells were aliquoted, plated and treated for 24h
- Treatments included vehicle alone, MDP or Imhotex test compounds
- After 24h, supernatants were removed and stored at -80°C until analysis
- The monocytes remaining in plate wells were tested for viability
Assays for IL-6 were carried out on the samples to assess response to potential stimulation of NOD2. A complex block randomisation process was used in the shuffling of supernatants from the one volunteer per plate monocyte assay to the IL-6 assay to limit bias. In addition, following removal of the supernatants, the cells were checked for viability to ensure that none of the compounds were cytotoxic.
In total, 27 volunteers attended RxCelerate and provided a sufficient blood sample for analysis:
- 5 control subjects
- with no NOD2 mutations
- 22 subjects with Crohn’s disease
- 6 with no NOD2 mutations
- 5 with the Leu1007fs mutations
- 5 with the Arg702Trp mutations
- 6 with the Gly908Arg mutations
Results
IL-6 response to vehicle
There is no significant difference among the subject groups in IL-6 response to vehicle alone, but you can see that there are very big differences between subjects.
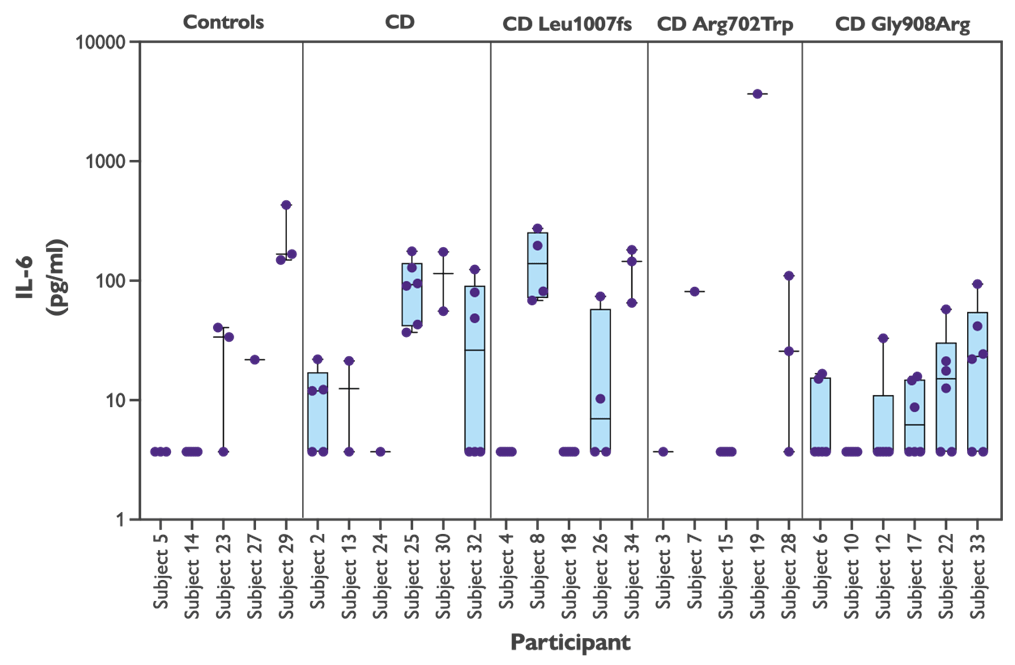
Figure 1: IL-6 in response to vehicle alone
Treatment with NOD2 agonists
All of the NOD2 agonists tested, both the natural activator MDP and the novel Imhotex compounds, increased IL-6 production by the monocytes. There is no difference in the IL-6 response from monocytes treated with any of the agonists between control subjects and volunteers with Crohn’s disease (or CD). There are some small differences among the Crohn’s disease subjects with different genotypes, but the differences are not statistically significant.
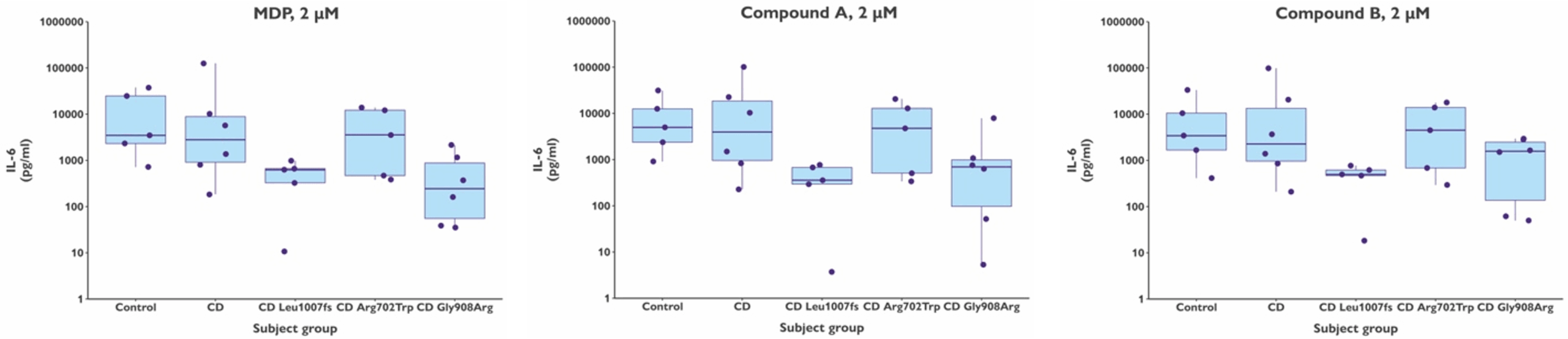
Figure 2: Response of volunteer monocytes to treatment with NOD2 agonists. Each graph shows treatment with a single concentration of compound. IL-6 concentrations are shown after subtraction of IL-6 produced in cells treated with vehicle alone.
Subject-specific effects
The graphs above show a very similar response to both the natural NOD2 agonist MDP and the Imhotex test compounds by the volunteer monocytes. When plotted by volunteer, rather than by subject group (see next Figure) one can see that there are very clear subject-specific effects. For any given subject, whichever NOD2 agonist they are treated with, the IL-6 response is very similar. But the person-to-person differences are substantial.
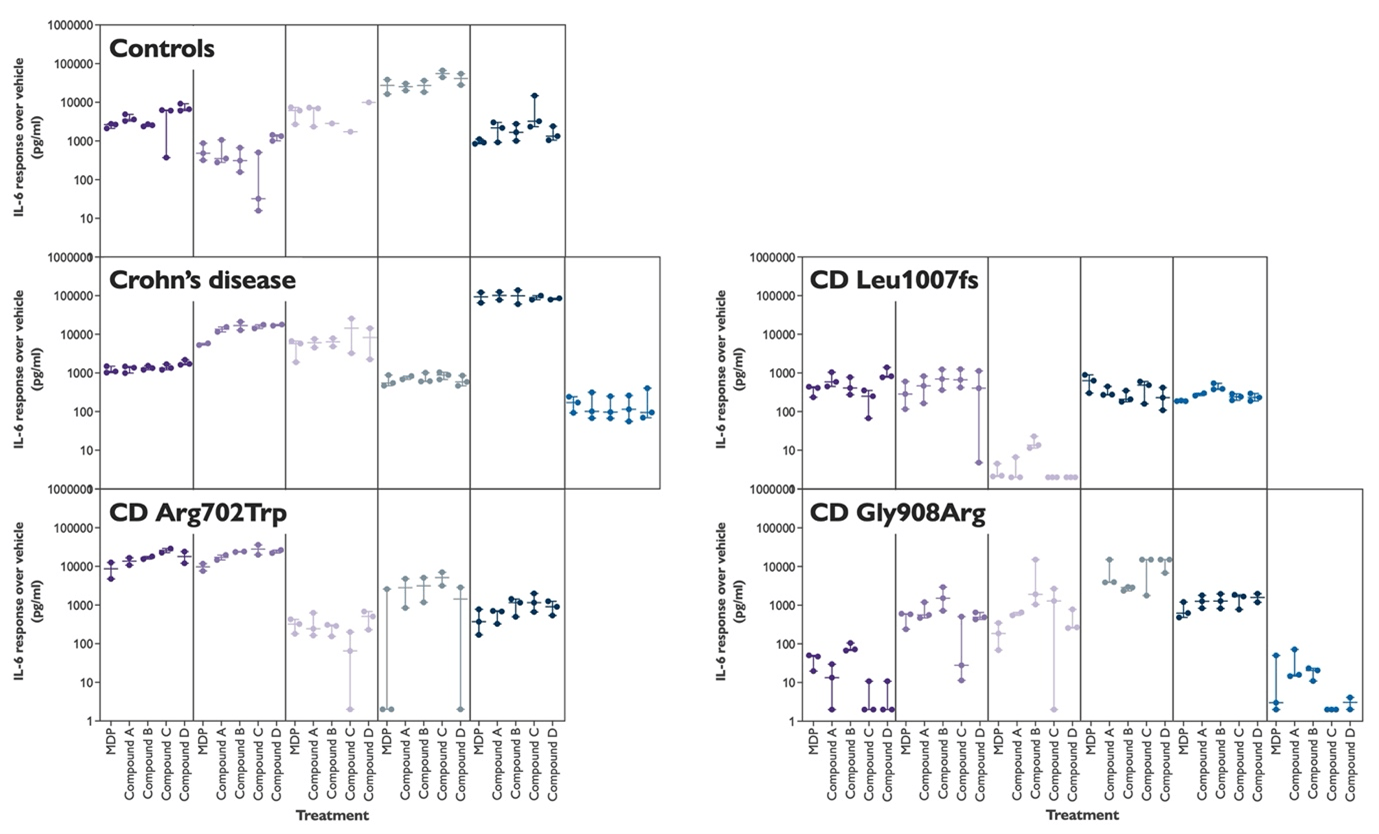
Figure 3: Response of volunteer monocytes to treatment with a range of different NOD2 agonists, both natural and new. Each box contains the data from a single subject.
Summary and conclusions
Overall, the study ran efficiently, with timely recruitment and well-coordinated execution of visits, sample work, and analysis.
We found a low level of IL-6 was released by cells without stimulation by NOD2 agonists, with values varying among subjects but unrelated to Crohn’s or NOD2 genotype.
Secondly, and most importantly, we have shown that in this study both the natural NOD2 agonist MDP and the Imhotex compounds designed to activate NOD2 had very similar effects to each other, both triggering a marked IL-6 release from ex vivo monocytes in a 24-hour period.
There was no difference in IL-6 release from monocytes isolated from control subjects and volunteers with Crohn’s disease. Furthermore, there was no statistically significant difference in the IL-6 response from monocytes isolated from volunteers with Crohn’s disease with different NOD2 genotypes.
This suggests that release of IL-6 in response to NOD2 agonists – at least in monocytes under these conditions – is not impaired in patients with Crohn’s disease, even those with NOD2 genotypes thought to reduce its activity.
There is, however, a striking person-to-person difference in the amount of IL-6 released following stimulation with NOD2 agonists. We see differences of up to 10,000-fold between subjects. This variability is currently unexplained. Age and sex are not related to the response suggesting that maybe other genetic or environmental factors may be at play.
Find out more about RxCelerate’s Experimental Medicine service – designing and delivering complex in vitro human translational studies.
Case Studies
Read through our case studies to see how our recent innovations are delivering impact, from rapid small molecule screening to antibody discovery campaigns and bespoke preclinical animal models.
