Generation of Antibodies to PD-1
Introduction
We have engineered and constructed the Galaxy® antibody library that underpins our proprietary platform. Our ‘glass spleen’ approach incorporates the learnings of the in vivo selection process and allows us to access a far greater fraction of relevant antibody space. Based on the principle of ‘smart randomness’, the Galaxy® library harnesses the incredible diversity found within human B cells in a focused way, maximising the proportion of correctly folded functional antibodies.
This process results in potent antibodies without the need for excessive mutational load. The Galaxy® platform is geared for generation of ‘clinic ready’ antibodies straight out of the library. Furthermore, the design provides the potential to rapidly generate bispecifics without the need for any special technology or engineering.
At RxBiologics our capabilities extend to screening for desired specificity and cross-reactivity, NGS analysis, in silico developability and immunogenicity screening, profiling for functional activity, antibody production, affinity determination, epitope and biophysical characterisation.
Case study:
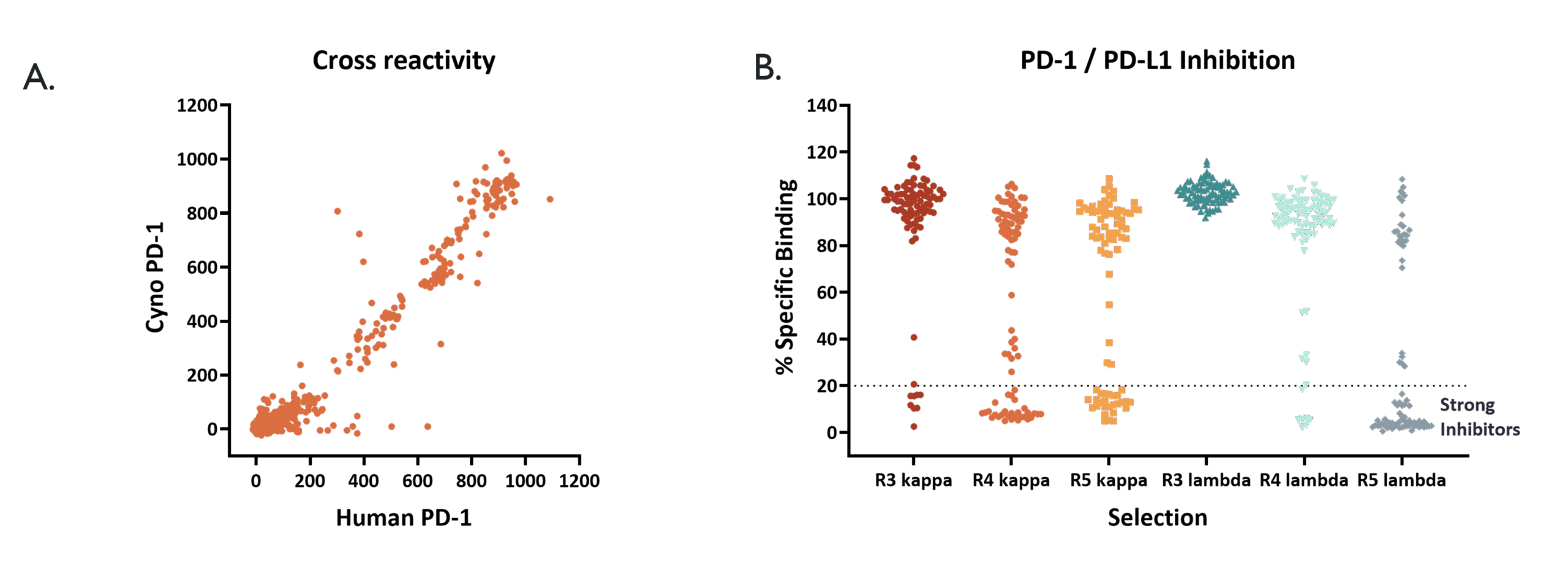
After phage selections, clones were expressed as Fab fragments and tested in a TR-FRET direct binding assay (A.), showing human/cyno PD-1 cross-reactivity, and a competition assay (B.), with many showing a large reduction in % specific binding due to inhibition of the PD-1/PD-L1 receptor ligand complex.
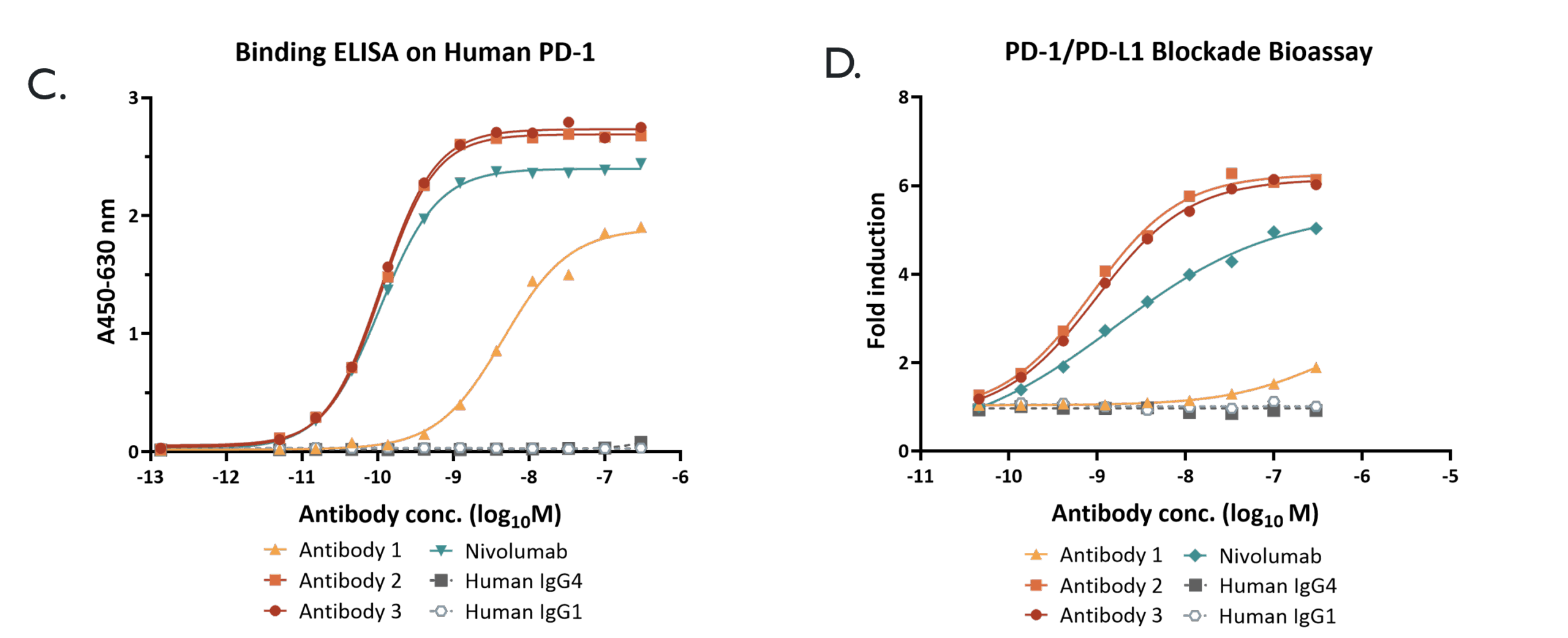
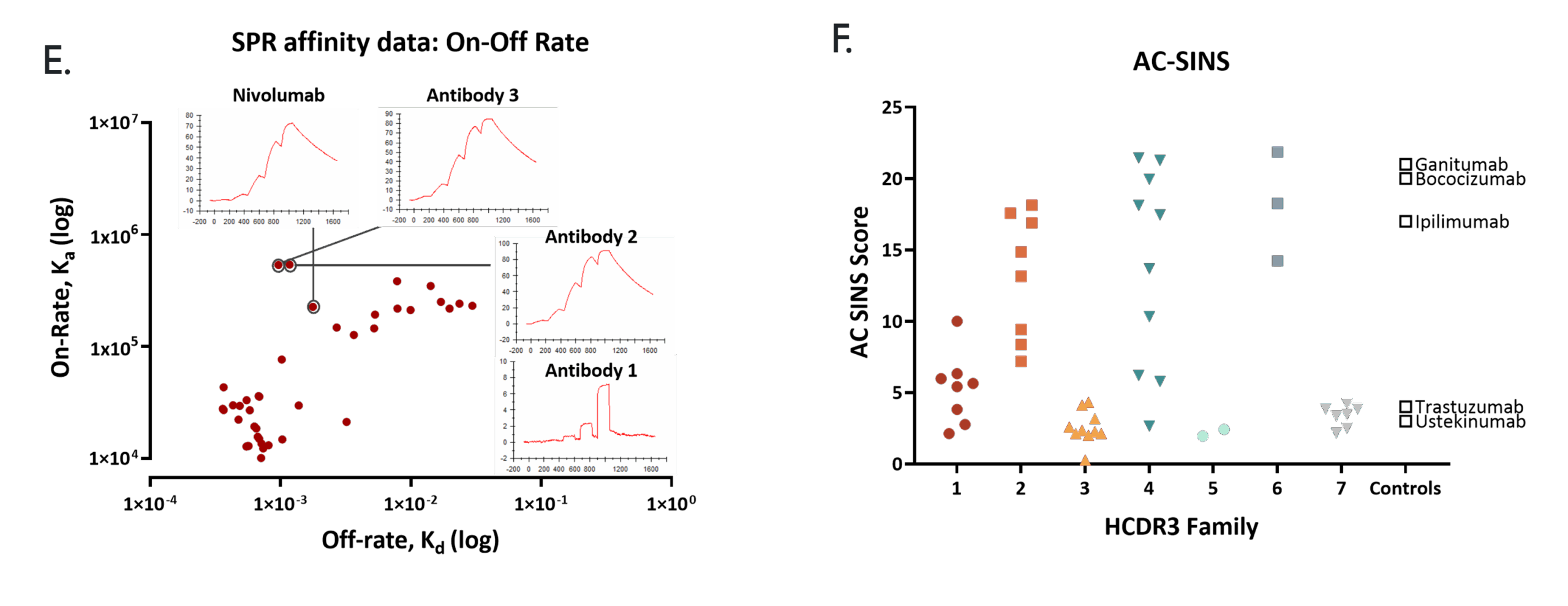
SPR affinity data (E.) shows the on- and off-rates for a panel of IgG’s, highlighting the improvement in affinity antibodies 2 & 3 over the parental antibody 1. Affinity-Capture Self-Interaction Nanoparticle Spectroscopy (AC-SINS) data (F.) predicts self-association propensity. Our approach gives a choice of clones within each HCDR3 family so the most developable can be selected.
Properties of Antibodies from the Galaxy® Platform
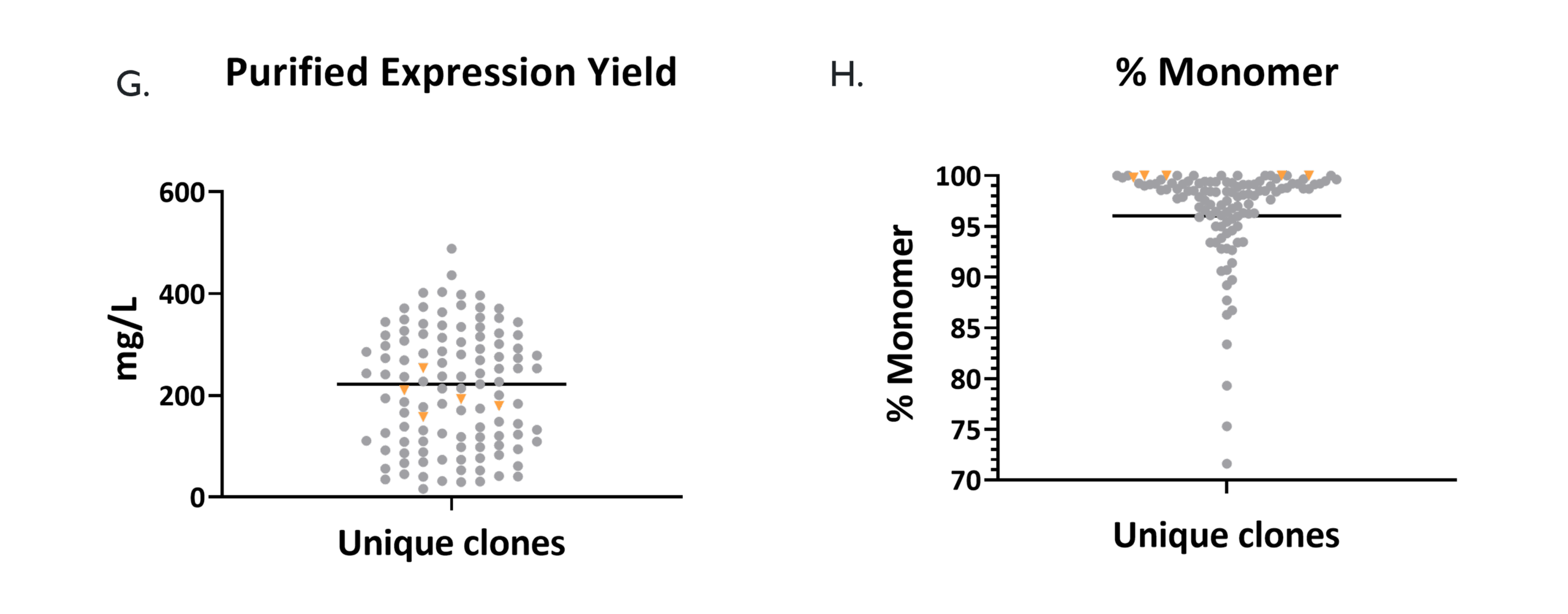
Hits to a variety of target classes were produced as IgG1 (grey). The expression yields (G.) and percentage monomer fraction (H.) were compared to antibodies in the clinic (orange).
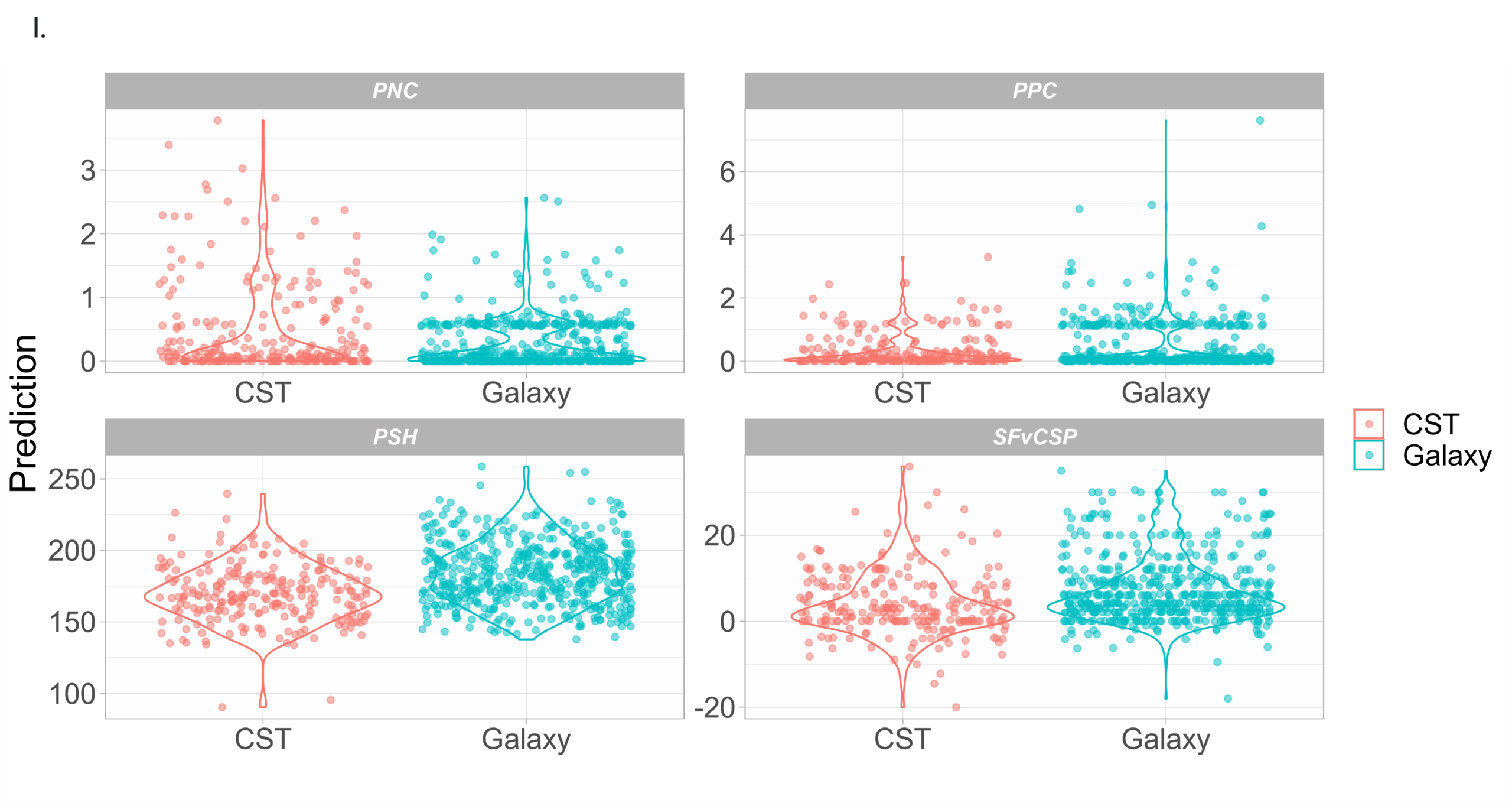
In Silico Developability
We also carried out an in silico developability analysis (I.) using the Therapeutic Antibody Profiler (TAP) software (Raybould M et al. 2019). A large proportion of antibodies, straight out of the Galaxy® platform, have properties comparable to clinical stage antibody therapeutics (CST).
Summary
Our whole process is built around the principle of ‘right first time’, efficiently delivering high quality biologics with drug-like properties. ProsaRx extends our service to provide computer aided drug discovery that incorporates AI/ML. With our parent company RxCelerate we are able to support an entire drug programme, small molecule, protein or antibody, from concept to clinic.
To find out more, visit RxBiologics.com
